Alzheimer’s disease research is at the forefront of scientific inquiry, as experts work tirelessly to unravel the complexities of this debilitating condition. With an estimated 7 million Americans currently affected, innovations are not just necessary but imperative; Beth Stevens, a prominent neuroscientist, has been instrumental in shifting paradigms regarding microglial cells, the brain’s immune system. These cells play a critical role in brain health by removing damaged tissue and supporting neuronal functions, yet emerging studies indicate that their malfunction may contribute to the development of neurodegenerative diseases like Alzheimer’s. Stevens’ groundbreaking findings pave the way for novel Alzheimer’s treatments and biomarkers, offering hope for earlier detection and intervention. As the aging population continues to grow, the urgency for effective research and strategies to combat this health crisis only intensifies.
Investigating the complexities of Alzheimer’s disease is essential in the quest for better understanding and management of neurodegenerative disorders. Groundbreaking research led by innovators like Beth Stevens sheds light on the crucial role of microglial cells, which serve as the brain’s first line of defense against degeneration. By studying how these immune cells interact within the brain, scientists aim to uncover new pathways to develop effective Alzheimer’s treatments that could significantly improve patient outcomes. As the prevalence of cognitive disorders increases, the demand for advanced understanding and proactive approaches becomes more pressing. This exploration into the brain’s immune system not only furthers our scientific knowledge but also holds the promise of revolutionizing care for millions affected by Alzheimer’s.
Understanding Microglial Cells and Their Role in Alzheimer’s Disease
Microglial cells are essential components of the brain’s immune system, tasked with maintaining brain health. Their primary function is to survey the central nervous system, identifying and eliminating dead or damaged cells. In the context of Alzheimer’s disease research, these cells have been found to have a dual role. On one hand, they promote brain health by pruning unnecessary synapses, but on the other hand, aberrant microglial activity can contribute to neurodegenerative diseases. This complicated balancing act is crucial for understanding how Alzheimer’s develops and progresses, and it highlights the potential of targeting microglia in new therapeutic approaches.
Recent studies led by neuroscientist Beth Stevens have accelerated our understanding of how microglial dysfunction could lead to Alzheimer’s. Her work emphasizes the importance of microglia not only in routine brain maintenance but also in their potential to exacerbate neuronal damage when their pruning capabilities go awry. Stevens’ findings suggest that these immune cells are not just passive players; instead, they actively shape the neural landscape, indicating that targeted interventions on microglial function may one day yield effective treatments for Alzheimer’s and other neurodegenerative conditions.
Innovative Approaches to Alzheimer’s Treatments Through Curiosity-Driven Research
Curiosity-driven research has become a cornerstone in the fight against Alzheimer’s disease. Beth Stevens illustrates how fundamental research laid the groundwork for innovative treatments. By exploring the intricate biology of microglial cells, researchers like Stevens have uncovered mechanisms that allow these immune cells to regulate synaptic activity and potentially influence the onset of Alzheimer’s. Insights gained from studying the visual systems of mice, for instance, provide pathways for understanding human neurodegenerative diseases, pushing the boundaries of traditional biomedical research.
This approach is crucial, especially as Alzheimer’s cases are projected to double in the coming years, necessitating new strategies for prediction and intervention. Stevens’ research not only aims to develop new medications but also strives to identify early biomarkers that can detect Alzheimer’s before it manifests severely. Such proactive measures might transform care models, alleviating the burden on healthcare systems while improving the lives of millions affected by neurodegenerative diseases.
The Significance of Basic Science in Alzheimer’s Disease Research
The contributions of basic science to understanding Alzheimer’s disease cannot be overstated. Beth Stevens’ path illustrates the vital role that fundamental research plays in developing practical applications. Often, initial investigations might not seem directly related to disease at first glance, as seen in her studies on the visual systems of mice. Yet, the insights gained from such research have significant implications for understanding complex diseases like Alzheimer’s. Basic science fuels innovation by uncovering foundational knowledge that leads to breakthroughs in treatment and diagnostics.
Moreover, the support of federal agencies has been instrumental in propelling this research forward. Funding from the National Institutes of Health and other organizations allows scientists to explore various aspects of disease, ultimately leading to discoveries that impact human health. This interplay between curiosity-driven investigation and funding underscores the importance of nurturing a research environment where novel ideas can flourish and contribute to the fight against Alzheimer’s and related neurodegenerative diseases.
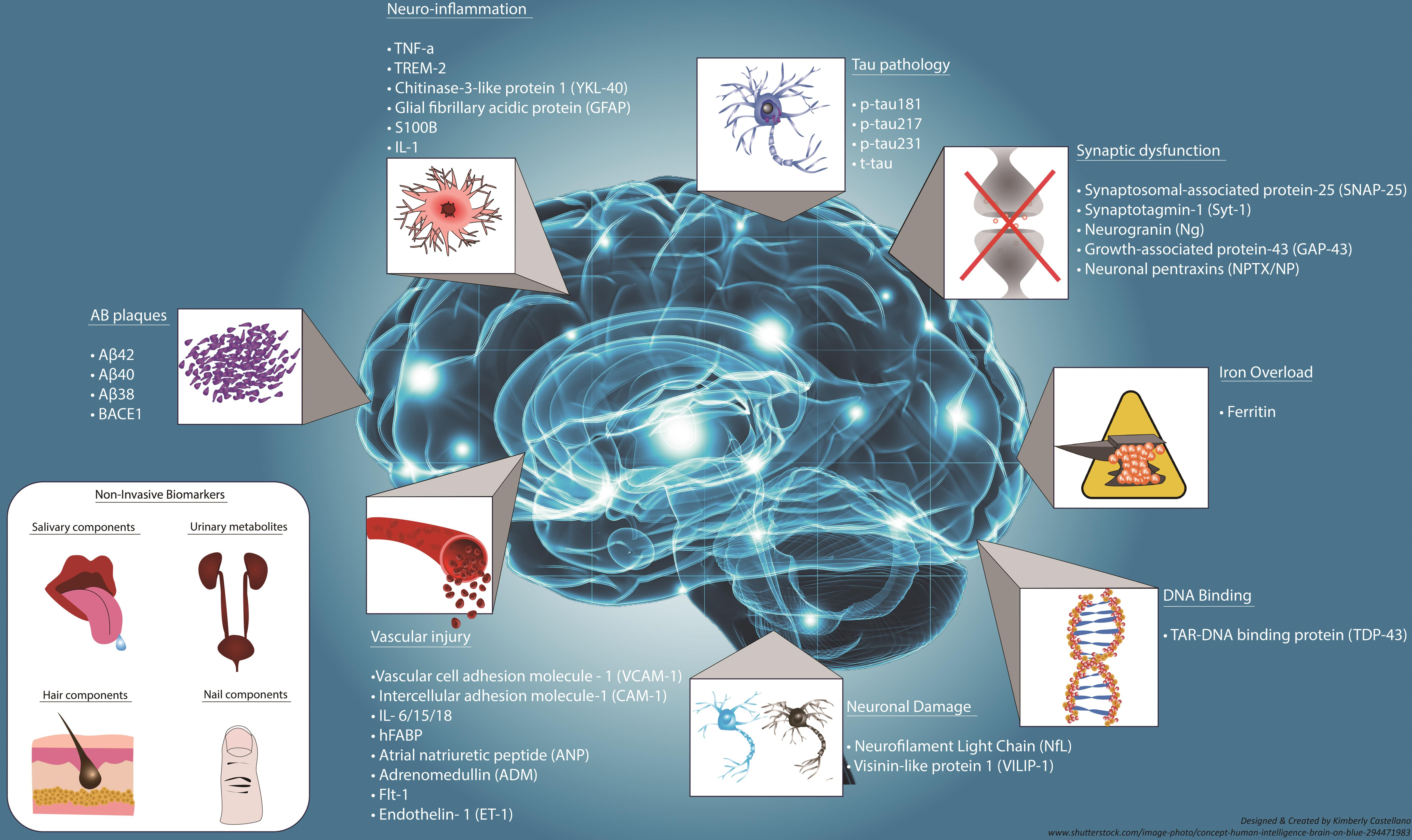
Exploring New Biomarkers for Early Detection of Alzheimer’s Disease
The race for early detection of Alzheimer’s disease has gained momentum with advancements in research on microglial cells and their role in neurodegeneration. Identifying biomarkers that signal the onset of Alzheimer’s at its early stages is critical for effective intervention strategies. Stevens’ research aims to develop these biomarkers by understanding how microglial activity can serve as a hallmark of pathological changes in the brain associated with Alzheimer’s.
This shift towards early diagnosis could revolutionize the treatment landscape for Alzheimer’s, potentially allowing for preventative measures before significant cognitive decline occurs. By leveraging the knowledge obtained from studying microglia, future therapies may not only target existing symptoms but also proactively address the underlying biological mechanisms driving disease progression.
The Future of Neurodegenerative Diseases Research
As the global population ages, the prevalence of neurodegenerative diseases, including Alzheimer’s, continues to increase, calling for urgent and innovative research solutions. The findings from Beth Stevens and her colleagues underscore the importance of interdisciplinary efforts to unravel the complexities of these conditions. By integrating insights from various scientific domains, researchers can better understand the pathophysiology of Alzheimer’s and develop multifaceted approaches to care.
This collaborative atmosphere is beneficial in painting a comprehensive picture of how neurodegenerative diseases operate, pushing the boundaries of conventional understanding. As research progresses, there is hope that new treatments will emerge, harnessing the knowledge gained from studying microglial functionality and other aspects of brain immune responses, ultimately improving outcomes for those affected by Alzheimer’s and related disorders.
Beth Stevens: A Trailblazer in Alzheimer’s Disease Research
Beth Stevens has emerged as a leading figure in Alzheimer’s disease research, known for her groundbreaking work on microglial cells and their intricate role in neurotransmission and brain health. Her recognition as a MacArthur ‘genius’ reflects the profound impact of her findings, which highlight how these immune cells participate in both healthy brain function and the progression of neurodegenerative diseases. Stevens’ innovative approach combines basic science with the potential for discoveries that translate into effective treatments.
By continuing to push the envelope of scientific inquiry, Stevens and her lab are at the forefront of identifying how disruptions in microglial functions contribute to Alzheimer’s. The transformative potential of her research emphasizes the need for ongoing support and funding in the scientific exploration of neurodegenerative diseases, as every discovery can lead to new therapeutic avenues that might one day alleviate the burden of Alzheimer’s on millions of families.
Challenges in Researching Alzheimer’s Therapy Development
Developing therapies for Alzheimer’s disease presents numerous challenges, underscoring the complexity of neurodegenerative diseases. One major hurdle is translating findings from fundamental research into clinical applications. Many promising discoveries, like those relating to microglial cells, often require extensive validation processes before they can be considered viable treatment options. Stevens points out that while the potential for treatment breakthroughs exists, the path is often laden with regulatory and logistical obstacles that can delay progress.
Moreover, the biological diversity of Alzheimer’s disease means that a one-size-fits-all approach may not be effective. Researchers must account for variations in disease presentation and progression among individuals, necessitating customized treatment strategies rooted in robust scientific inquiry. Addressing these challenges requires collaboration across disciplines, with a unified goal to expedite the development of effective therapies stemming from foundational research.
The Role of Federal Funding in Alzheimer’s Research
Federal funding plays a pivotal role in advancing Alzheimer’s disease research, providing critical resources for scientists like Beth Stevens. The NIH and other agencies are instrumental in supporting studies that explore the underlying causes of Alzheimer’s and the pathophysiology of neurodegenerative diseases. This financial backing is particularly vital in the early stages of research, where initial findings can inform larger-scale studies and lead to transformative discoveries that improve our understanding and treatment of Alzheimer’s.
Sustained federal investment in neuroscience is crucial, as it fosters an environment of scientific exploration and collaboration. By prioritizing funding for Alzheimer’s-related research, agencies can not only support established scientists but also encourage emerging researchers to tackle the pressing challenges of neurodegenerative diseases, ultimately aiming to deliver new hope for those affected and their families.
Impacts of Alzheimer’s on Society and Caregiver Burden
As Alzheimer’s disease prevalence increases, its impact on society becomes significantly more pronounced. The rising number of cases places a heavy burden on healthcare systems, particularly in terms of providing ongoing care for individuals suffering from neurodegenerative diseases. The shift in the age demographics within the U.S. population, compounded by rising care costs, signals an urgent need for effective treatments and support systems to mitigate these challenges.
Moreover, caregivers bear a considerable burden, often facing emotional, physical, and financial strains while caring for loved ones with Alzheimer’s. Recognizing the challenges faced by caregivers is crucial for developing supportive policies and services that not only prioritize patient care but also ensure caregivers have access to the resources they need. Improving the quality of life for both patients and caregivers hinges on holistic approaches that consider the societal implications of Alzheimer’s.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells act as the brain’s immune system, patrolling for signs of illness and injury. In Alzheimer’s disease research, studies by scientists like Beth Stevens show that these cells are crucial for clearing out damaged cells and pruning synapses. However, aberrant microglial pruning can contribute to neurodegenerative diseases like Alzheimer’s, making them a significant focus for potential treatments and interventions.
How does Beth Stevens’ research influence treatments for Alzheimer’s disease?
Beth Stevens’ research on microglial cells has transformed our understanding of the brain’s immune system in the context of Alzheimer’s disease. Her findings highlight how improper synaptic pruning by microglia can lead to neurodegenerative diseases, paving the way for innovative Alzheimer’s treatments that target these cellular processes and improve patient outcomes.
What are the implications of aberrant pruning in neurodegenerative diseases?
Aberrant pruning by microglial cells, as studied in Alzheimer’s disease research, can disrupt normal neural communication and contribute to the progression of neurodegenerative diseases. Understanding this process is vital, as it allows researchers to develop targeted therapies aimed at correcting improper microglial functions to potentially halt or slow the progression of Alzheimer’s disease.
How is Alzheimer’s disease linked to the immune system in the brain?
Recent Alzheimer’s disease research emphasizes the role of the brain’s immune system, particularly microglial cells, in maintaining neural health. Aberrant activity of these immune cells can lead to neuroinflammation and contribute to the development of Alzheimer’s disease, highlighting the importance of the brain’s immune response in neurodegenerative processes.
What are some new biomarkers being researched for early detection of Alzheimer’s disease?
In the realm of Alzheimer’s disease research, scientists are developing new biomarkers that can help detect the disease earlier. These biomarkers are often based on the activity and health of microglial cells, as well as other neuroinflammatory markers, enabling timely interventions and improved treatment outcomes for patients.
What funding sources support Alzheimer’s disease research like that of Beth Stevens?
Alzheimer’s disease research, such as that conducted by Beth Stevens, is often supported by federal agencies like the National Institutes of Health (NIH). These agencies provide vital funding that allows researchers to explore critical questions related to microglial cells and their role in neurodegenerative diseases.
| Key Points | Details |
|---|---|
| Role of Microglia | Microglia act as the brain’s immune system, clearing dead cells and pruning synapses. |
| Aberrant Pruning | Improper pruning by microglia is linked to Alzheimer’s, Huntington’s, and other disorders. |
| Research Impact | Beth Stevens’ research aids in developing drugs and biomarkers for neurodegenerative diseases. |
| Future of Alzheimer’s Cases | Projected doubling of cases by 2050, highlighting an urgent need for research. |
| Funding Sources | The National Institutes of Health provided vital support for Stevens’ research. |
Summary
Alzheimer’s disease research is progressing rapidly, as demonstrated by the groundbreaking work of neuroscientist Beth Stevens. Her study on microglial cells reveals their critical role in brain health and the potential consequences of their dysfunction in neurodegenerative diseases such as Alzheimer’s. With an increasing number of cases expected in the coming decades, continued investment in Alzheimer’s disease research is essential for developing new treatments and improving patient outcomes. Stevens’ findings not only spotlight the importance of microglia but also reinforce the value of curiosity-driven basic science in addressing complex health issues.