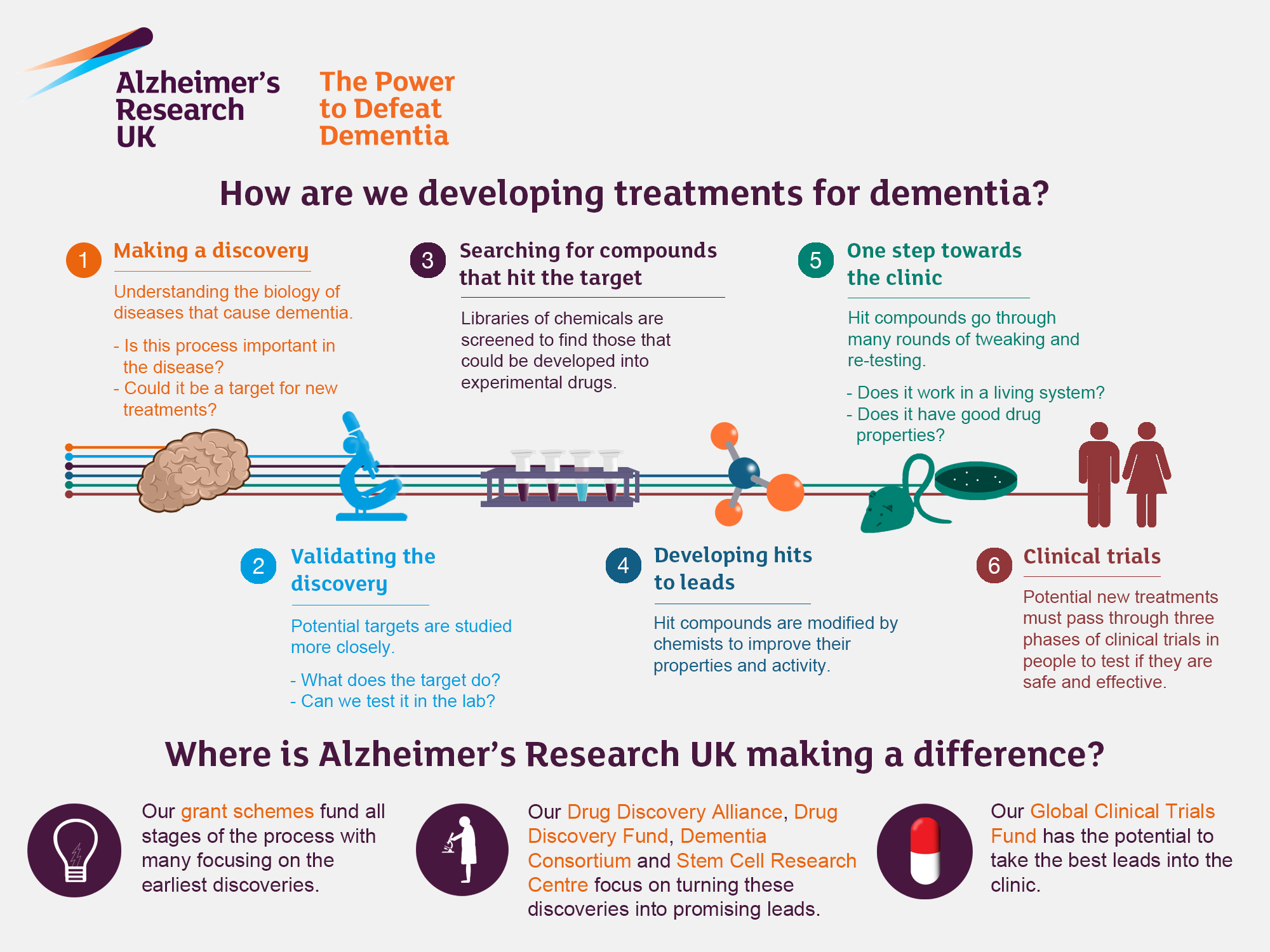
Alzheimer’s research is at the forefront of scientific inquiry as we seek to unravel the complexities of this debilitating neurodegenerative disease. Led by pioneering scientists like Beth Stevens, who studies microglial cells, the brain’s immune system, this field has significantly deepened our understanding of how synapses are pruned and maintained. Abnormal microglial activity can lead to damage within the brain, contributing to Alzheimer’s and other neurodegenerative diseases. The innovative work being conducted at the Stevens Lab is not only illuminating the underlying biology of Alzheimer’s treatment options but also paving the way for new biomarkers that could revolutionize early detection. As we delve deeper into the intricate relationship between microglial function and neuronal health, the potential for groundbreaking advancements in combating this condition grows ever more promising.
Research focused on Alzheimer’s, a progressive brain disorder impacting cognitive function and memory, is revolutionizing our understanding of brain health. This branch of science examines critical elements such as the role of brain immune mechanisms, particularly microglial cells, which are essential for maintaining neural integrity and responding to damage. Under the leadership of visionaries like Beth Stevens, efforts are being directed toward identifying how these immune responses can lead to pathological changes associated with Alzheimer’s and similar diseases. Alternative avenues of inquiry promise to unlock novel approaches for diagnosing and treating conditions that currently have no cure. By investigating immune system pathways and their effects on neuronal behavior, we inch closer to safer, effective interventions for millions affected by cognitive decline.
The Role of Microglial Cells in Alzheimer’s Disease
Microglial cells play a crucial role in maintaining brain health by acting as the primary immune cells of the central nervous system. They constantly surveil the brain, identifying and responding to signs of disease or injury. Their ability to prune synapses is essential for normal neural development and function, but an imbalance in their activity can lead to detrimental effects. In the context of Alzheimer’s disease, abnormal microglial activity has been implicated in damaging healthy neural connections, exacerbating cognitive decline. Researchers are increasingly finding that understanding these cells’ behavior can shed light on the underlying mechanisms of neurodegenerative diseases.
Recent studies from the Stevens Lab have highlighted the link between microglial cells and abnormal synaptic pruning in Alzheimer’s. These findings suggest that premature or excessive pruning by microglia may contribute to the loss of critical neural circuits in affected individuals. Developing drugs that can modulate microglial activity could potentially slow the progression of Alzheimer’s disease. This approach represents a paradigm shift in how we view the treatment of neurodegenerative disorders, encouraging a focus on the brain’s immune response as a therapeutic target.
Innovations in Alzheimer’s Research by the Stevens Lab
The Stevens Lab’s groundbreaking work has opened new avenues in Alzheimer’s research, especially in understanding how microglial cells interact with neuronal health. By elucidating the mechanisms of synaptic pruning, the lab has laid a foundation for identifying new biomarkers for Alzheimer’s disease. These biomarkers could facilitate earlier diagnosis and enable targeted interventions. Furthermore, the Stevens Lab’s investigations into the immune dynamics of the brain have significant implications for the broader category of neurodegenerative diseases, suggesting potential treatment pathways not just for Alzheimer’s, but also for conditions like Huntington’s disease.
Another significant breakthrough from the Stevens Lab is the development of novel therapeutic strategies aimed at restoring normal microglial function. By leveraging insights gained from basic science, Stevens and her team have initiated projects aimed at producing drugs that enhance microglial activity, promoting their ability to clear dysfunctional synapses without harming healthy neural connections. This innovative approach could lead to the first effective treatments for Alzheimer’s, addressing not only symptoms but also the root causes of this devastating disease.
Understanding How the Brain’s Immune System Works
The brain’s immune system is a complex network that functions differently than the standard immune response seen in other organs. Microglial cells are central to this system, uniquely equipped to respond to various stimuli in the neural environment. Their roles extend beyond mere surveillance; they actively participate in sculpting the brain’s architecture. Understanding how microglia adapt to different challenges is crucial in unraveling their influence on neurodegenerative diseases. This knowledge can guide researchers in manipulating microglial responses to treat conditions like Alzheimer’s.
Recent advances in neuroscience underscore the importance of the brain’s immune system in relation to overall neural health. Studies that focus on microglial biology have revealed that these cells are not only defenders but are also essential for maintaining homeostasis in brain circuits. When functioning correctly, microglial cells support synaptic connections, promote neurogenesis, and facilitate recovery from injuries. However, dysregulation can lead to increased inflammation and neuronal damage, integral to the pathology of diseases like Alzheimer’s. Ongoing research aims to develop interventions that can restore appropriate microglial function, paving the way for breakthroughs in treatment.
Future Directions in Alzheimer’s Treatment Research
As research on Alzheimer’s disease advances, the focus is shifting toward innovative treatments that target the brain’s immune response. The insights gained from understanding microglial cells have laid a critical foundation for developing new therapeutic strategies. By identifying specific pathways where microglial dysfunction contributes to synaptic loss, researchers can craft drugs designed to rectify these imbalances. This approach not only aims to alleviate symptoms but also seeks to delay or prevent the onset of cognitive decline in individuals at risk.
Collaboration within the scientific community is essential to push forward the development of these treatments. Multi-disciplinary teams, including molecular biologists, pharmacologists, and neuroscientists, are working toward creating comprehensive strategies that address Alzheimer’s disease holistically. By combining knowledge from various fields, there’s hope to accelerate the discovery of effective therapies that leverage the brain’s immune system to combat neurodegenerative diseases. This evolution in Alzheimer’s research promises a future where effective treatments are not just a dream but an attainable reality.
The Impact of Federal Funding on Alzheimer’s Research
Federal funding has been a cornerstone for advancing Alzheimer’s research, providing vital resources needed for groundbreaking studies like those conducted by the Stevens Lab. Grants from the National Institutes of Health (NIH) have empowered researchers to explore complex questions about brain health and immune function. This financial support is crucial, particularly in areas that may not immediately yield a clear outcome but hold great potential for future discoveries that can reshape our understanding of neurodegenerative diseases.
Stevens emphasizes that the foundation of their inquiry into microglial behavior and its implications for Alzheimer’s are directly tied to this federal backing. The ability to conduct long-term studies is essential for developing and testing hypotheses related to brain immune function and disease. As funding continues to support innovative research, it bolsters the possibility of translating discoveries into impactful treatments, ultimately improving the lives of millions affected by Alzheimer’s and similar disorders.
Translating Basic Research into Clinical Applications
The journey from basic research to clinical application is often a long and complex one. In the field of Alzheimer’s research, the insights obtained from studying microglial cells are beginning to inform potential treatment strategies. The Stevens Lab’s exploration of how these cells manage neuronal health is paving the way for translating findings into therapeutic interventions. By understanding the underlying mechanisms that lead to abnormalities in synaptic pruning, researchers are taking critical steps toward developing drugs that can mitigate the effects of Alzheimer’s.
The promise of translating basic findings into real-world applications is an exciting prospect for both scientists and patients alike. Each discovery increases our understanding of the biological processes involved in neurodegenerative diseases and provides critical knowledge that can lead to the development of effective treatments. The work being done at institutions like the Stevens Lab represents a vital link in this chain, embodying the essential nature of curiosity-driven science that ultimately aims to improve patient outcomes in Alzheimer’s and other neurodegenerative disorders.
The Significance of Synaptic Pruning in Development and Disease
Synaptic pruning is a fundamental process in neural development, critical for the maturation of healthy brain circuits. In a healthy brain, microglial cells ensure that synapses that are no longer necessary are efficiently eliminated, allowing for the refinement of neural networks. However, in conditions such as Alzheimer’s disease, this process can become dysfunctional, leading to excessive or premature pruning. Understanding the dual role of microglial cells in both promoting healthy development and contributing to disease is central to addressing neurodegenerative disorders.
Research conducted by the Stevens Lab has uncovered how synaptic pruning relates to the neurological landscape in Alzheimer’s. By studying the mechanisms that drive microglial activity, scientists can better understand how disruptions in this process may lead to cognitive decline. The delicate balance of synaptic pruning is essential not just in development but also in adult brain function. Dysregulation of this process opens avenues for innovative treatments aimed at correcting the course of neurodegenerative diseases, providing hope for patients and families.
Holistic Approaches to Alzheimer’s Disorder Management
Managing Alzheimer’s disease requires a holistic approach that encompasses not only medical intervention but also lifestyle modifications and supportive therapies. As research delves deeper into the role of microglial cells in brain health, it becomes clear that strategies must be designed to address various aspects of the disease. Initiatives that focus on diet, cognitive engagement, and physical activity can help maintain brain function and may mitigate the progression of symptoms for individuals diagnosed with Alzheimer’s.
Moreover, caregivers play an essential role in the management of Alzheimer’s, benefiting from resources and training that help them understand the disease better. Educating families about the importance of a coordinated care plan involving medical management, emotional support, and lifestyle adjustments is critical. As our understanding of Alzheimer’s disease evolves, embracing a comprehensive approach that integrates scientific research with practical care strategies can greatly enhance the quality of life for those affected.
The Future of Drug Development in Alzheimer’s Research
The future of drug development in Alzheimer’s research holds immense potential, especially with the advancements made in understanding microglial function. By targeting the immune pathways involved in Alzheimer’s, pharmaceutical research is on the brink of discovering groundbreaking therapies. Scientists are refining strategies to enhance or inhibit microglial activity, which could halt the progression of neurodegenerative diseases early on. The goal is to create not just symptomatic treatments, but potential disease-modifying therapies that can protect brain health.
Incorporating innovative approaches, such as gene therapy or immunomodulation, further demonstrates the dynamic landscape of Alzheimer’s treatment research. By creating drugs that can specifically adjust microglial responses, researchers hope to develop agents that not only treat Alzheimer’s symptoms but also restore the brain’s ability to manage fluency in normal cognitive processes. The extensive focus on drug discovery marks a hopeful horizon for Alzheimer’s patients, reflecting the dedication of researchers committed to changing the narrative of this complex disease.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are vital components of the brain’s immune system, playing a crucial role in Alzheimer’s research. They patrol the brain for signs of disease or injury, helping to clear dead cells and prune synapses. In Alzheimer’s disease, abnormal microglial activity is linked to neurodegeneration, making these cells a key focus in developing new treatments and biomarkers for Alzheimer’s.
How does the research at the Beth Stevens lab contribute to understanding Alzheimer’s disease?
The Stevens lab, based at Boston Children’s Hospital and the Broad Institute, has significantly advanced our understanding of Alzheimer’s disease through its research on microglial cells. By uncovering how abnormal pruning by microglia contributes to neurodegenerative diseases, the lab is paving the way for new biomarkers and treatment strategies aimed at improving care for individuals with Alzheimer’s.
What advancements in Alzheimer’s treatment stem from studies of the brain’s immune system?
Recent advancements in Alzheimer’s treatment are largely influenced by studies of the brain’s immune system, particularly investigations into microglial cells. Research conducted by Beth Stevens and her team emphasizes how these cells impact synaptic pruning and neurodegeneration, potentially leading to novel therapeutic approaches for Alzheimer’s and related neurodegenerative diseases.
What insights have been gained from studying microglial cells in neurodegenerative diseases?
Studying microglial cells has provided critical insights into the mechanisms underlying various neurodegenerative diseases, including Alzheimer’s. Research has shown that while microglia serve protective roles, their dysregulation can result in detrimental effects like excessive synaptic pruning, contributing to disease progression and offering new targets for therapeutic intervention.
How does curiosity-driven science impact Alzheimer’s research?
Curiosity-driven science significantly impacts Alzheimer’s research by fostering innovative discoveries that might not have immediate applications. As illustrated by Beth Stevens, this foundational research on microglial cells and the brain’s immune system leads to critical advancements in understanding Alzheimer’s and ultimately informs the development of effective treatments.
| Key Point | Details |
|---|---|
| Innovation in Microglia Research | Beth Stevens has reshaped the understanding of microglial cells as crucial elements of the brain’s immune system. |
| Role of Microglia | They clear damaged cells and prune synapses, affecting brain health. |
| Link to Alzheimer’s | Abnormal pruning by microglia is implicated in Alzheimer’s and other neurodegenerative diseases. |
| Impacts of Research | Stevens’ research supports the development of new biomarkers and medicines for Alzheimer’s. |
| Importance of Basic Science | Basic and curiosity-driven research is essential for scientific breakthroughs in Alzheimer’s. |
Summary
Alzheimer’s research is advancing significantly, thanks in large part to the insights gained from the work of pioneers like Beth Stevens. Her groundbreaking studies on microglial cells have illuminated their critical role in brain health and disease, highlighting how abnormal microglial activity can contribute to Alzheimer’s and other neurodegenerative disorders. This progress not only promises to enhance our understanding of these diseases but also opens pathways for innovative treatments and biomarkers that could ultimately improve the lives of millions affected by Alzheimer’s.